Alcohol to Mesylate
[(MeSO2)2O]
Examples:
Example 1
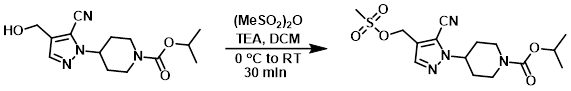
To a solution of the SM (75 mg, 0.24 mmol) in anhydrous DCM (1 mL) was added TEA (0.1 mL, 0.74 mmol). The mixture was cooled in an ice bath and treated with methanesulfonic anhydride (62 mg, 0.34 mmol). The reaction mixture was removed from the ice bath and stirred for 30 min. The mixture was quenched by the addition of sat aq NaHCO3. The layers were separated and the aq layer was further extracted with DCM (3x). The combined organics were washed with brine, dried (Na2SO4), and concentrated to provide the product as an oil which was taken forward without further purification. [75 mg]
[Patent Reference: WO2012069948, page 53, ![]() (3.9 MB)]
(3.9 MB)]
Example 2

To a solution of the SM (2.8 g, 14.0 mmol) in DCM (30 mL) at 0 C was added TEA (5.3 mL, 42.0 mmol) followed by methanesulfonic anhydride (3.7 g, 21.0 mmol). The reaction mixture was warmed to RT and stirred for 2 h. To the mixture was added sat aq NaHCO3 (100 mL). The layers were separated and the aq layer was further extracted with DCM. The combined organics were dried (MgSO4) and concentrated to provide the product. [3.8 g, 98%]
[Patent Reference: WO2016014463, page 63, ![]() (6.7 MB)]
(6.7 MB)]
