Aluminum Isopropoxide
Other Names:
Aluminium Isopropoxide
Aluminum triisopropoxide
Aluminum isopropylate
AIP
General Information:
Structure:
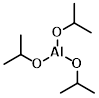
CAS Number: 555-31-7
Molecular Weight: 204.25 g/mol
Appearance: White solid
Melting Point: 128-133 C
The main use of aluminum isopropoxide is in Oppenauer oxidations and Meerwein-Ponndorf-Verley (MPV) reductions. The two reactions are part of a reversible process, the direction of which depends on whether excess acetone or excess IPA are present, congruent with Le Chatelier’s principle. For example, in the presence of excess acetone the reaction is driven to the right towards the ketone product (Oppenauer oxidation).
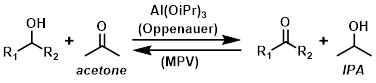
Common Uses:
Reagent in Oppenauer oxidations
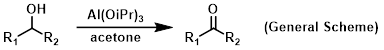
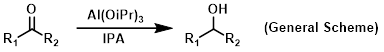
Reagent in Meerwein-Ponndorf-Verley (MPV) reductions
Safety:
Aluminum isopropoxide is corrosive, moisture sensitive, and flammable.
References:
1) Burke, S. D.; Danheiser, R. L.; Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents
2) Wikipedia: Aluminium isopropoxide (link)
3) www.sigmaaldrich.com: Aluminum isopropoxide (link)
4) www.alfa.com: 14007 Aluminum isopropoxide, 98+% (link)
5) Kurti, L.; Czako, B.; Strategic Applications of Named Reactions in Organic Synthesis
