Aluminum Chloride
Other Names:
Aluminium chloride
Aluminium(III) chloride
General Information:
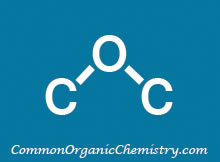
Structure:
AlCl3
CAS Number: 7446-70-0 (anhydrous)
Molecular Weight: 133.34 g/mol (anhydrous)
Appearance: Colorless to yellow solid
Chemical Formula: AlCl3
Melting Point: 190 C (anhydrous)
Aluminum chloride (AlCl3) is used as a Lewis acid catalyst for a variety of reactions. The most well-known application of AlCl3 is in Friedel-Crafts reactions. Friedel-Crafts alkylations generally only require a catalytic amount of AlCl3, whereas Friedel-Crafts acylations require greater than one equivalent of AlCl3. This is because the Lewis acidity of AlCl3 results in strong complexes with carbonyl groups (the acylating agent).
Common Uses:
Reagent for Friedel-Crafts alkylations
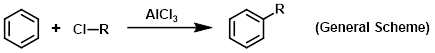
Reagent for Friedel-Crafts acylations
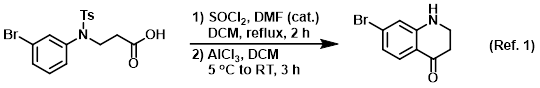
Procedure excerpt:
. . . A mixture of AlCl3 (3.40 g, 25.18 mmol) in DCM (20 mL) was cooled to 5 C, then the acid chloride (prepared above) in DCM (10 mL) was added dropwise . . .
Safety:
Aluminum chloride reacts violently with H2O. Aluminum chloride fumes in air and should therefore be stored in a tightly sealed container and protected from moisture.
References:
1) Patent Reference: WO2015089337, page 207, ![]() (17.5 MB)
(17.5 MB)
2) Wikipedia: Aluminium chloride (link)
3) www.sigmaaldrich.com: Aluminum chloride (link)
4) www.alfa.com: 12298 Aluminum chloride, anhydrous, Reagent Grade (link)
5) Reich, H. J.; Rigby, J. H.; Handbook of Reagents for Organic Synthesis, Acidic and Basic Reagents
6) Kurti, L.; Czako, B.; Strategic Applications of Named Reactions in Organic Synthesis