Acetyl Chloride
Other Names:
Ethanoyl chloride
Acyl chloride
General Information:
Structure:
![]()
CAS Number: 75-36-5
Molecular Weight: 78.49 g/mol
Appearance: Colorless liquid
Chemical Formula: CH3COCl
Melting Point: -112 C
Boiling Point: 52 C
Density: 1.104 g/mL at 25 C
Acetyl chloride is a simple acyl halide commonly used to introduce acetyl groups onto amine or alcohol functionalities of substrates. Acetyl groups are sometimes used as protecting groups for amines. A common alternative to acetyl chloride for acetylation reactions is acetic anhydride.
Common Uses:
Reagent for the acetyl protection of amines
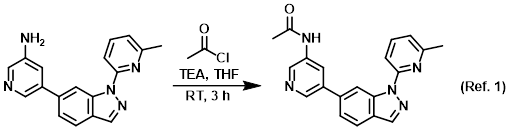
Procedure excerpt:
To a mixture of the SM (65 mg, 0.22 mmol) and TEA (67 mg, 0.66 mmol) in THF (5 mL) was added acetyl chloride (34 mg, 0.44 mmol) at RT. The reaction mixture . . .
Reagent for the acetylation of alcohols
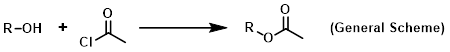
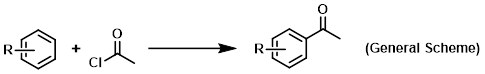
Reagent in Friedel-Crafts acylations
Safety:
Acetyl chloride is a corrosive, volatile liquid.
References:
1) Patent Reference: WO2016011390, page 63, ![]() (20.2 MB)
(20.2 MB)
2) Wikipedia: Acetyl chloride (link)
3) www.sigmaaldrich.com: Acetyl chloride (link)
4) www.alfa.com: 43262 Acetyl chloride, 99+% (link)
5) Kurti, L.; Czako, B.; Strategic Applications of Named Reactions in Organic Synthesis
