Chlorination
(NCS)
Examples:
Example 1
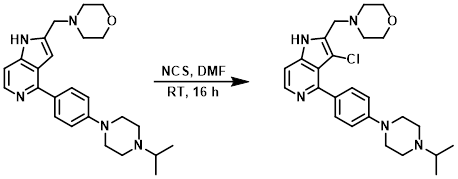
To a solution of the SM (0.10 g, 0.23 mmol) in anhydrous DMF (2.3 mL) was added NCS (0.038 g, 0.29 mmol) at RT. The reaction mixture was stirred at RT for 16 h. The excess solvent was removed in vacuo, and the obtained residue was diluted with ice-H2O, then extracted with EtOAc (3 x 20 mL). The combined organics were washed with brine (25 mL), dried (Na2SO4), and concentrated. The residue was purified by Prep TLC (10% MeOH/DCM) to provide the product as a yellow solid. [0.037 g, 35%]
[Patent Reference: WO2015088045, page 215, ![]() (10.3 MB)]
(10.3 MB)]
Example 2
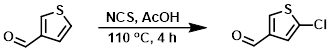
A solution of the SM (20.0 g, 178.3 mmol) and NCS (23.8 g, 178.3 mmol) in AcOH (180 mL) was stirred at 110 C for 4 h. After cooling to RT, the mixture was diluted with EtOAc (120 mL), washed with H2O (3 x 100 mL), sat aq NaHCO3 (2 x 50 mL), brine, dried (Na2SO4), and concentrated to provide the product as a yellow solid which was taken forward without further purification. [8.0 g, 31%]
[Patent Reference: WO2015140133, page 110, ![]() (11.7 MB)]
(11.7 MB)]
Example 3
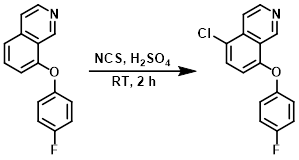
NCS (0.67 g, 2.0 mmol) was added portionwise to a solution of the SM (0.6 g, 2.5 mmol) in H2SO4 (3 mL) at 0 C. The reaction mixture was then warmed to RT and stirred for 2 h. After completion (by TLC), the mixture was cooled to 0 C and quenched with ice-cold H2O. The precipitate was filtered and washed with cold n-hexane to provide the product. [0.5 g, 73%]
[Patent Reference: WO2016021742, page 100, ![]() (7.7 MB)]
(7.7 MB)]
Example 4
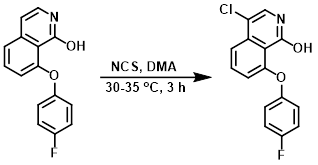
NCS (0.22 g, 1.66 mmol) was added portionwise to a solution of the SM (0.42 g, 1.66 mmol) in DMA (15 mL) at 0 C. The reaction mixture was stirred at 30-35 C for 3 h. After completion (by TLC), the mixture was cooled to 0 C and quenched with ice-cold H2O. The precipitate was filtered and washed with cold n-hexane to provide the product. [0.4 g, 83%]
[Patent Reference: WO2016021742, page 102, ![]() (7.7 MB)]
(7.7 MB)]
